Dr. Thomas Wong

Dr. Thomas Wong
Office: Science 408
Phone: (718) 631-6939
Email: TWong@qcc.cuny.edu
EDUCATION
- Ph.D., Medicinal Chemistry, St. John’s University, Queens, New York
- MS, Organic Chemistry, St. John’s University, Queens, New York
- MA, Biology, Hofstra University, Hempstead, New York
- BS, Chemistry, York College, Jamaica, New York
- AS, Science, LaGuardia Community College, Long Island City, New York
COURSES TAUGHT
Queensborough Community College
- CH-127: Introductory Chemistry
- CH-151: General Chemistry I
- CH-152: General Chemistry II
Queens College
- CH-251: Organic Chemistry 1 Lecture
- CH-252: Organic Chemistry 2 Lecture
TEACHING PHILOSOPHY
To prepare students to become independent thinkers and doers for graduate school, dental school, veterinary, nursing school, and medical school.
RESEARCH EXPERIENCE and INTEREST
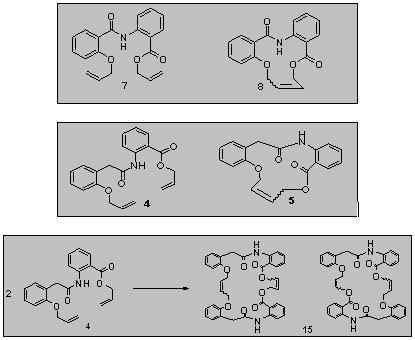
For my Master’s project, compounds were synthesized using ring-closing metathesis (RCM). The RCM reaction resulted in the formation of monomer rings and one dimer ring. Compounds 4 and 7 are dienes. Compounds 5 and 8 are the monomer rings formed from the RCM reaction. Compound 4 also produced compound 15 (dimer ring).
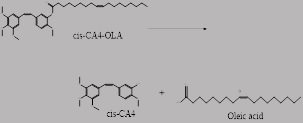
For my doctoral research, 16 conjugates of combretastatin A4 were produce, potential anticancer drugs. Conjugates are compounds linked to a carrier moiety, in this case, a polyunsaturated fatty acid. Once the conjugate reaches the target, the active drug and carrier moiety are cleave. Illustration of conjugate of CA4 linked to Oleic Acid which is separated into it components after reaching the target site. Combretastatin A4 is a natural product, which binds to tubulin that prevents cancer cells from undergoing mitosis. Thus, cancer cells do not replicate.
PRESENTATION
- David P. Brown and Thomas Wong, “Synthesis of Novel Macrocyclic Compounds in the Development of New Chemical Entities for Drug Discovery Research”, The 40th Middle Atlantic Regional Meeting (MARM) of the American Chemical Society, Queensborough Community College, May 18, 2008 (poster presentation).
PUBLICATIONS
- David P. Brown, and Thomas Wong, “A Synthetic Study of Dibenzo-Aromatic Macrolactams”, Heterocycles, 2010, 81(1).
- Thomas Wong, Silpa Narayanan, David P. Brown and Zhe-Sheng Chen, “Synthesis and Cytotoxicity Studies of Stilbene Long-Chain Fatty Acid Conjugates”, Journal of Natural Products, 2020, 83, 5, 1563-1570.