Sideris Research Group
Undergraduate Research Students
Updated February 2025
Current Students
- Abdullah Husain4
- Pedrocia De-Sosoo3
- Sherlin Rosales Caamano2
Former Students
- Daletsi Reyes2
- Ahmed Tafsir2
- Thrisha Mae Lumor2
- Tyra Volney
- Zachary Avrutis1
- Emmanuel Agunloye
- Sung Hwan Ahn1
- Hui Zhu
- Sindy Ferreiras
- Krystal Lee
- Yueli Chen
- Heera Choe
- Nicole Yu
4Co-mentored with Dr. Rebecca Coles (National Security and Nonproliferation Department, Brookhaven National Laboratory) and Dr. Sharon Lall-Ramnarine (Chemistry Department) as part of the "Developing Next Generation Radiation Safety Professionals" (DNGRSP) Program.
3Co-mentored with Dr. Andrea Mattera (National Nuclear Data Center, Brookhaven National Laboratory) and Dr. Sharon Lall-Ramnarine (Chemistry Department) as part of the "Developing Next Generation Radiation Safety Professionals" (DNGRSP) Program.
2Co-mentored with Dr. Sharon Lall-Ramnarine (Chemistry Department), as part of the "Developing Next Generation Radiation Safety Professionals" (DNGRSP) Program.
1Co-mentored with Prof. Michael Lawrence (Engineering Technology Department) and Dr. Paul Marchese (Physics Department).
Research Highlights
Our group synthesizes and characterizes materials for various energy storage devices such as lithium ion batteries and supercapacitors. We primarily use powder X-ray diffraction (PXRD), nuclear magnetic resonance (NMR) spectroscopy, and scanning electron microscopy (SEM) to investigate the local and long-range structure of these materials.
We have collaborated extensively with the Greenbaum Laboratory at CUNY Hunter College on several projects. We also have access to several CUNY research facilities, such as the New York Structural Biology Center (NYSBC) and the Advanced Science Research Center (ASRC).
Scroll down for some promising, preliminary results obtained by our team!
Fabrication of a Laser-induced Graphene Capacitor
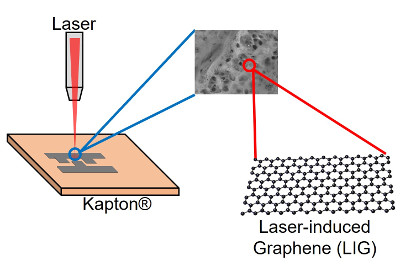
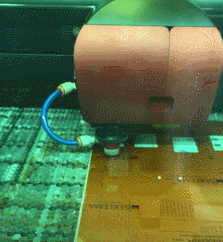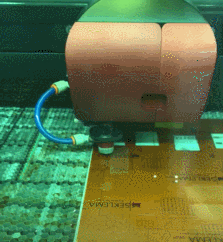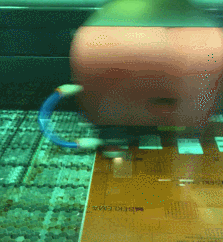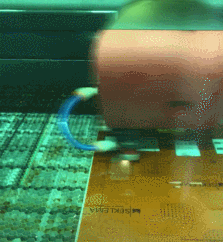
By carefully controlling the lasing parameters of a laser cutter in the Engineering Technology Department, we can convert specific areas of a plastic sheet (Kapton) into an electrically conductive form of carbon known as graphene. The laser-induced graphene (LIG) that is made from this process acts as an electrode for an energy storage device known as a capacitor. The computer-controlled, high-accuracy laser beam positioning in the laser cutter allows us to reproducibly create these graphene electrodes in a variety of designs.
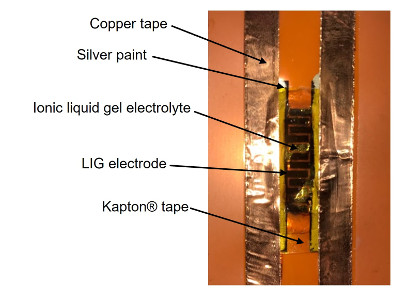
The photograph above depicts a fully assembled capacitor on a sheet of Kapton plastic, with in-plane LIG electrodes and an ionic liquid gel electrolyte that was prepared in our lab. The LIG was patterned into interdigitated electrodes, with each "microelectrode" having a length of 4.1 millimeters and a width of 1 millimeter. The spacing between neighboring microelectrodes was approximately 300 micrometers. Silver colloidal paint and copper tape were added to the common areas of both electrodes to facilitate electrical testing.
Evidence of Fe-O-P Bonds In Ionothermal Reactions
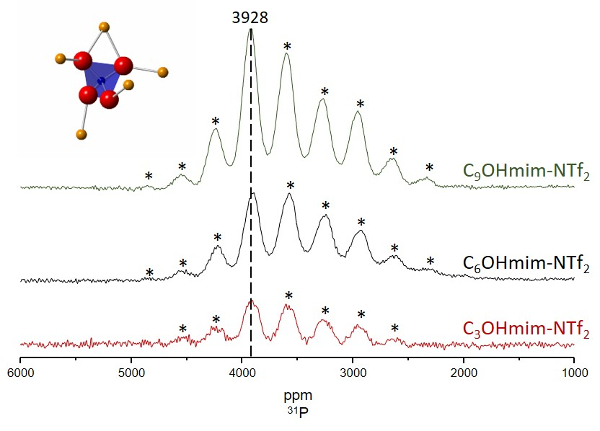
Ionothermal syntheses of LiFePO4 from salt precursors were attempted in three ionic liquids: 1-(3-hydroxypropyl)-3-methyl imidazolium bis(trifluoromethylsulfonyl)amide, 1-(6-hydroxyhexyl)-3-methyl imidazolium bis(trifluoromethylsulfonyl)amide, and 1-(9-hydroxynonyl)-3-methyl imidazolium bis(trifluoromethylsulfonyl)amide, or C3OHmim-NTf2, C6OHmim-NTf2, and C9OHmim-NTf2 respectively. The 31P magic angle spinning (MAS) NMR spectra of the solids obtained after the reactions are depicted above. Spectra were collected at 7 T using a Hahn echo sequence and a sample spinning rate of ~35 kHz. Asterisks (*) denote spinning sidebands.
The phosphorus local environment in LiFePO4 is shown on the top-left of the spectrum. Phosphorus (P) atoms are in blue, oxygen (O) atoms are in red, and iron (Fe) atoms are in orange. The Fermi Contact Interaction, which describes the through-bond transfer of electron density from a paramagnetic center (the Fe atom) to a probe nucleus (the P atom), is primarily responsible for the very large shifts (~3928 ppm) observed. These shifts are consistent with the formation of Fe-O-P bonds.
New Ionic Liquid for Ionothermal Synthesis
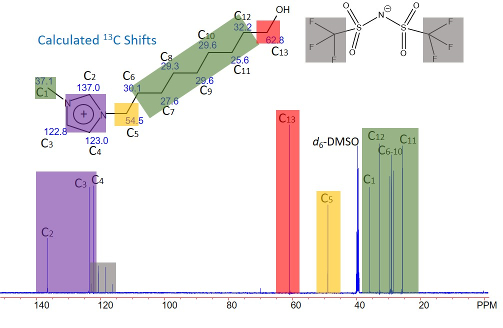
The figure depicts the 1H-decoupled 13C NMR spectrum of 1-(9-hydroxynonyl)-3-methyl imidazolium bis(trifluoromethylsulfonyl)amide (abbreviated as C9OHmim-NTf2), a new ionic liquid for our ionothermal reaction study. The structure of the cation and anion, along with the estimated 13C shifts, are shown above the spectrum. A series of ionic liquids can be readily made in high yield using a two-step, microwave-assisted synthesis.
Surfactant-Assisted Hydrothermal Reactions
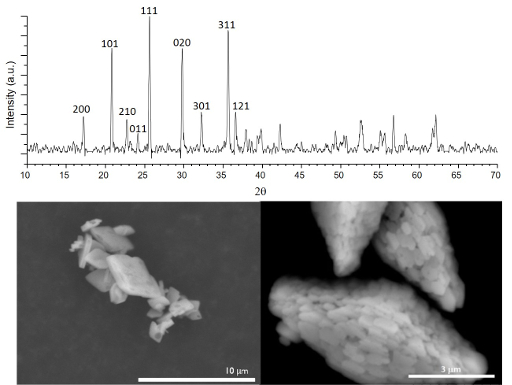
LiFePO4 crystallizes in the Pnma spacegroup (No. 62) with a~10.329 Å, b~6.007 Å, c~4.691 Å. The top figure depicts a typical PXRD pattern we obtain of LiFePO4 that is prepared by a hydrothermal synthetic route. Select reflections are indexed. Below the diffraction pattern are SEM images of LiFePO4 particles synthesized without any surfactant (left), and in the presence of sodium dodecylbenzenesulfonate (right), an anionic surfactant.
Lithium Ion Dynamics in Solid Electrolytes
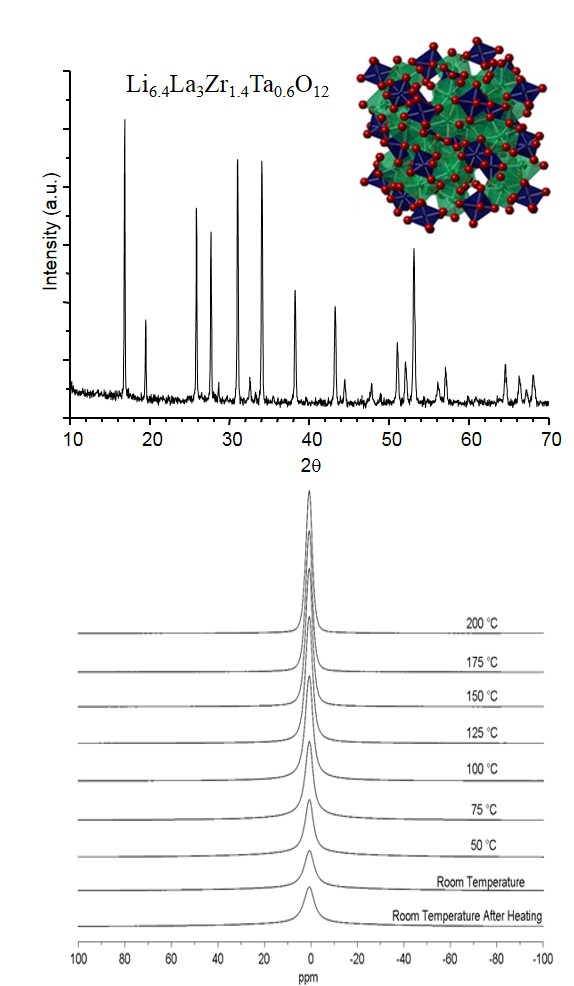
The powder X-ray diffraction pattern of Li6.4La3Zr1.4Ta0.6O12 prepared by a high temperature solid state reaction is shown in the top figure. The relatively intense and sharp reflections indicate a highly crystalline, well-ordered sample.
A polyhedral representation of the framework of the garnet-like material is depicted on the top-right; the lithium (Li) atoms are omitted for clarity. Lanthanum (La) atoms are shown in green, Zirconium (Zr) and Tantalum (Ta) atoms are in blue, and oxygen (O) atoms are in red.
Variable temperature solid state 7Li NMR spectra of Li6.4La3Zr1.4Ta0.6O12 are shown in the bottom figure. The measurements were made at 7 T using a static probe. Motional narrowing of the lithium signal is observed as the sample is heated to 200 °C due to Li+ conduction through the garnet-like framework.



