SYNTHETIC GEMSTONES
A synthetic gemstone has the same properties
as a natural stone; that is, all the physical, chemical, and optical characteristic
are identical. The only difference is that synthetic gemstones are created
in a laboratory. So remember they are not minerals, since they are not
made by nature. If a substance is made to resemble a gemstone, but
does not share its characteristics, it is called a simulant.
Many people get confused, and unscrupulous dealers sell
simulants under the name synthetic. Simulants can be almost as difficult
to detect as synthetic gemstones, but unlike synthetics specialized detectors
can reveal differences in the physical properties and detect the simulant;
this would not generally work for a synthetic. One exeption is DeBeers'
system for detecting synthetic diamonds.
One of the simplest tools for detecting simulants is a
refractometer, which measures a property related to the speed of light
in the stone called the refractive index of the
stone. Glass is the most common simulant and will have a different
refractive index than the gem it simulates. Refractive index measurement
using a refractometer is nondestructive and can even be applied to some
stones that are mounted in small pieces of jewelry.
There are synthetic versions of many popular gemstones
and many of them have been around for a long time. Older synthetics are
simple for gemologists to detect; they were often too good (no flaws).
Some modern synthetic gemstones are more difficult to detect. It takes
experience and some suspicion. A jeweler or gemologist can usually
detect synthetics by their unusual inclusions or growth features that are
not found in a natural stones. As well, certain characteristics of some
synthetic stones, such as most early synthetic diamonds made by General
Electric (GE) behaving as semiconductors, are rare in natural stones.
Thus be skeptical of something that seems too good or unusual to run across
on an average day.
Why Make Synthetic Gems?
Some of the most important synthetic stones and simulants are mentioned
below. Mostly these are produced because they are inexpensive and replace
a natural stone. As an example, synthetic ruby is used as a gemstone, but
synthetic quartz, which does have valuable industrial applications, is
seldom used as a gemstone because natural quartz is abundant and inexpensive.
Colored varieties of quartz may be more desirable for synthesis.
Some synthetic stones are quite valuable; for example, synthetic diamonds
are selling for about 2/3 the cost of natural ones of the same quality.
Because of limitations in production and the demand of the
consumer for natural products, synthetics are never as valuable as natural
stones. One example of a limitation is that very large synthetic diamonds
have not yet been grown in gem quality and thus do not yet compete for
this market. But even this will change soon!
Yet synthetic gems continue to be made. One reason
for this is that color matching of natural gems may be expensive and time
consuming, while synthetic gems may be very easily matched. Another
reason is that some jewelry requires near flawless stones and this is a
cheaper way to get them. No simulant quite matches the color of an
emerald, but a synthetic may. Synthetic emeralds sell for about a
third the price of a natural stone. As well, some natural stones
are so rare that it would be impossible to get a great stone, for instance
natural alexandrite, yet a synthetic is easily obtained at a fraction of
the cost. Color, clarity, and rarity with economy, that is the ultimate
answer.
Commonly synthesized gemstones include: alexandrite, corundum,
diamond, emerald, opal, spinel, and the colored varieties of quartz.
Several can be made by the same process. The major synthetic products
and processes are discussed below.
Synthetic Corundum (and gems created by similar
methodology)
Flame fusion--this method perfected by August Victor
Lewis Verneuil (a French chemist who lived from 1856-1913) and is often called
the Verneuil method. Use of flame fusion predates the publication
of Verneuil's work in 1902. The first synthetic corundum sold as
ruby was referred to as "Geneva Ruby." It appeared on the market around
1885 and was sold as natural material at first. Gemologists soon
doubted Geneva rubies had a natural origin and Verneuil began making a
similar but superior product around 1888 using powdered aluminum oxide
(Al2O3)
to grow an elongate, rounded mass of corundum, somewhat carrot shaped, called a boule. Powdered
Al2O3
and
a coloring agent such as chromium are fused (melted) as they are dropped
through the flame of a hydrogen and oxygen torch. The apparatus used is similar
to a blow torch. Verneuil never patented his technique and many companies
have produced corundum using his method almost without modification
from what was done in 1888!
The corundum is grown on a seed or rod of synthetic corundum.
Boules up to several hundred carats (about an inch wide by 3 inches long)
are produced. Synthetic corundum gem material can have some properties
not seen in natural stones which allow for moderately easy detection.
One such property is curved growth lines that are visible with 10 times
magnification another is the presence of gas bubbles.
One reason that this method was very popular is that watch
bearings are made of corundum; the Swiss no longer needed to import corundum
for their bearings, but could grow it and fashion it in their factories
to make their famous jewel-bearing watches.
Though many companies used this to create gems, perhaps
the most important advances in this method were achieved by the Linde (pronounded
lindy) Air Company, Chicago. They invented methods to control the
growth direction of the boule's crystallography and also invented the synthetic
star sapphire in 1947. Other material made by this method includes:
rutile, spinel, and strontium titanate. Rutile and strontium titanate were
important as diamond simulants until cubic zirconium took over.
Crystal pulling (Czochralski process)--This method
is used extensively in the production of ruby, alexandrite, YAG, and other materials for lasers;
it is also used for gems including: corundum, alexanderite, and the diamond
simulants GGG (Gadolinium Gallium Garnet) and YAG (Yttrium Aluminum Garnet),
which have no natural counterparts.
A very important use is in the high-tech field where single
crystal boules (carrot-shaped rods) of materials used to make semiconductors
and other types of electronic wafers, such as those used for computer chips, are pulled using this method.
After a large boule is pulled (diameters up to about 10 inches or 24.5
cm), it is sliced to make the thin wafers.
To do the pulling, a melt (hot liquid) of the correct
composition is prepared in a heat resistant crucible that is usually heated using radio
frequency methods and a seed crystal is lowered into it. The temperature
is reduced to just above the melting point of the seed, and the seed is
then rotated and slowly lifted out of the melt. A boule-like rod
is created as the seed is pulled out. The method creates large, very
pure crystals. One unusual aspect of the Czochralski method is that
hollow tubes and other shapes can be manufactured using this method.
Hydrothermal method--this method uses a high temperature
and pressure vessel that acts like a pressure cooker. Al2O3
for corundum, SiO2
for quartz, or a combination of the ingredients needed for emeralds, are
dissolved in a hotter part of the vessel's chamber and reprecipitate/crystallize
on seed crystals in a cooler part of the chamber. Crystals are formed
rather than a boule and the material appears more natural. A platinum
or silver wire sticking through the crystal gives it away, but when the
synthetic material is cut away from the wire and the seed crystal, these
gems may be difficult to detect.
A lack of natural inclusions and perhaps flakes of metal
from the walls of the growth chamber of the vessel may indicate a synthetic
gem. Gems grown by this method include: emerald and other varieties
of beryl (for example that made by Linde Air Company and Tairus Ltd.),
quartz (made by many companies for its use in electronics and a few for
jewelry), corundum, and alexandrite.
Unlike the flame fusion and Czochralski methods, the hydrothermal
(and flux growth mentioned below) allow the growth of chemically complex
minerals that can not be formed by flame fusion or melting in Czochralski
methods. This is because the ingredients (constituents) are either
too volatile or not compatible at normal pressure and the high temperatures
needed for melting. However, if the constituents are dissolved in
a fluid in a high temperature/hotter part of the hydrothermal apparatus,
the fluid can then migrate and can cool and precipitate in a cooler region
of the same device. Thus dissolution of ingrediants and precipitation
of gems occur because of a temperature gradient. Temperature gradients
exist in the earth, and hydrothermal (hot water) veins often contain interesting
mineralization such as gold, silver, emeralds, etc. that were dissolved
in the fluid deeper in the Earth's interior where it is hotter and reprecipitated
as the hot water rises through the Earth's crust and cools.
Flux growth--This method is popular for ruby, emerald,
alexandrite, and other exotic substances such as GGG, a type of garnet.
Crystals are grown in a crucible (vessel or pot) that contains feed of
the same composition as the mineral to be grown plus flux. Flux is
a substance used to lower the melting point of the feed so that crystals
can be grown at temperatures below the normal melting point of the gem
material. Flux is also a name used for applications in welding and
soldering, but the meaning of the word is not quite the same as in gem
making. The crucible is often made of platimum that has a very high
melting point. Sometimes seeds of the substance that is being grown
are suspended in the melt, as in the Gilson method of creating emeralds.
Some gems, such as Ramaura Ruby (trade mark), are created by self-nucleation.
That is, no seed is added.
The flux dissolves the constituents and as the vessel
containing the flux is cooled, the mineral/gem of interest crystallizes.
If allowed to cool completely, the gems would be encased in the flux.
So the flux is either poured off or drained before it solidifies.
Not only is it important not to cool below the flux's freezing point, but
it is also important to cool very slowly to grow as large a crystal(s)
of the gem as possible.
Synthetic Diamond
High pressure and high temperature method (HPHT)--This
method is the classic method first used by GE in 1954 to create industrial
diamonds and led to the creation of gem grade diamonds by around 1970.
Russian scientists (Kiev Synthetic Diamond Research Institute, 1967) made
the first synthetic gem diamonds. Several gem diamonds have been
produced by companies including DeBeers and General Electric; however,
they were not an economic success. On the other hand, industrial
diamonds used in drill bits and saws have been profitably produced by these
methods for the last several decades.
These diamonds are grown under similar conditions to natural
stones, temperatures of around 1,500-3,000oC and pressure of
between 852,365 to1,500,000 pounds per square inch (58,000-100,000 atmospheres).
A metal is mixed with the carbon used to grow the diamond and acts as a
flux. The metal may be iron, nickel, etc. Some diamonds created
by this method have metallic inclusions and can be attracted by a strong
magnet.
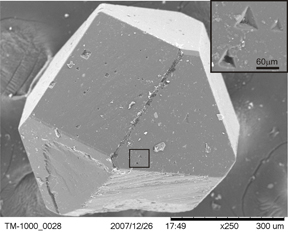
HPHT diamond displaying both cubic and octohedral forms in the
crystal. This diamond has
well developed crystal faces, but there are several etch(?) pits such
as those on the octohedral face,
which are magnified in the upper right corner of the photo. The
etch pits on the octohedral face
are triangular (octohedrons are made of 8 triangular faces. Those
on the cubic face are cubic. This
suggests a common method of formation.
Chemical vapor deposition method (CVD)--This method
is often used for depositing a thin layer of diamond on cutting tools or
other objects that need a hard surface. The term vapor implies a
gaseous phase and the gas used is often methane (CH4).
There is no need for high pressure, but temperatures are moderately high,
between 750-1,000oC.
Not all substances can be coated; it depends on their resistance to melting
and whether they form a carbide coating (good) or absorb the carbon (bad).
Most importantly, it is possible to grow a diamond on an existing diamond
substrate, and the rates of growth may be quite high. A gem quality
3-carat diamond may be grown in a few days. Because a single-crystal
seed diamond can be used to orient the growth, the diamond produced may
be grown with a controlled direction that allows for best cutting properties.
Opal
Opal is a hydrous (water-containing) form of silicon dioxide.
Opal's synthesis is achieved by creating uniformly sized, microscopic spheres
of silicon dioxide that are packed closely together, forming a solid mass.
The microscopic spheres act like a diffraction grating, breaking up light
into many colors. The exact methods used are proprietary and difficult
to achieve. However, it is clear that spheres of silicon dioxide
are created in an aqueous solution and settle slowly to the bottom of a
vessel. Once a mass of these spheres is created, heating or other
methods are used to consolidate the spheres into a mass.
Both white and black opal are made. The most prominent
company making synthetic opal is Gilson Opal (after Pierre Gilson's product
that first appeared on the market in 1974).
SIMULANTS
Substances, either real minerals or totally manmade,
can be called simulants if they are used to simulate another type of gemstone.
The most important gemstone simulated by many different materials is diamond.
Diamond simulants must have good fire like a true diamond, but many simulants
are overly fiery. Other simulants may be used for ruby, emerald,
sapphire, etc.
The most common simulant is glass. Colored glass
and glass with special characteristics can be used to simulate most gemstones.
For example, fiberlite (a fiber optics glass) is used to create simulants
of cat's eye (a stone showing chatoyancy), and Slocum stone a glass with
reflective foil simulates opal.
Cubic Zirconium (CZ)
At present, CZ is the most successful simulant for diamond.
However, moissanite (silicon carbide) is catching up.
Skull melting method--this method was invented
to produce cubic zirconium (CZ) from zirconium oxide powder.
Zirconium has such a high melting point (2,730oC)
that when melted, it cannot be contained in a crucible or other simple
containment vessel. The answer, simplicity itself, is to heat the
zirconium powder from the inside and use the remaining zirconium powder
surrounding the hot part as a containment vessel, with the tightly packed
unmelted powder acting as the containment walls. This is accomplished
by wrapping a radio frequency generator around the mass of powder.
The inside of the mass is heated and melts. Upon cooling the interior
portion is clear and inclusion-free. CZ is perhaps the best of the
diamond simulants. Because it is made by a simple method, it is relatively
inexpensive.
Moissanite (SiC)
Moissanite is named after the French chemist Ferdinand Henri
Moissan, a Nobel Laureate, who discovered natural silicon carbide (SiC)
in meteoric material at the Canyon Diablo Meterorite Crater in Arizona.
He won his Nobel for the isolation of the element fluorine in 1906.
He tried to make synthetic diamonds, but apparently failed though he did
grow silicon carbide.
Silicon carbide is also called carborundum and is used
as an abrasive. Carborundum is made by heating sand and coal to react
the coal's carbon with silicon in the sand. This process does not
produce gem quality material, though occassionally larger crystals may
be faceted. It has a hardness of 9.25 on Mohs' scale and has greater fire
than that of diamond.
Gem quality moissanite is made by a sublimation processes.
That is the constituents are vaporized and recrystallize by going directly
from a gas to a solid. This is done in a graphite crucible at a temperature
of around 2,500oC and the gases crystallize on a seed of SiC
to make a boule.
The production of colorless (perhaps with slight green
accents) SiC was a great techological achievement. Because moissanite
is not found in this form in nature, it is called a simulant here.
Because it is difficult to make, moissanite is expensive, though considerably
cheaper than diamond, which it simulates. The future for SiC in the
gem industry is uncertain. It may surpass cubic zirconium, but it
is unclear at this moment what will happen.
Return to the Biological Sciences and Geology
Website